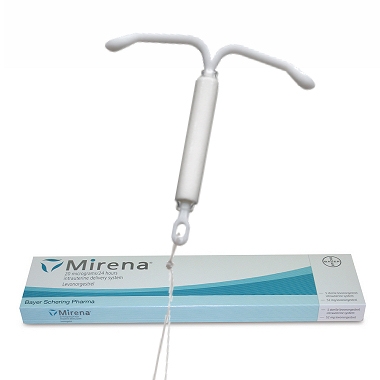
In 2000, the pharmaceutical giant, Bayer Healthcare Pharmaceuticals Inc., released its long-lasting method of birth control, the Mirena IUD (intrauterine device). In 2009, the U.S. Food and Drug Administration (FDA) additionally approved Mirena to treat heavy menstrual bleeding.
The Mirena IUD is a popular birth control device manufactured by Bayer Healthcare Pharmaceutical. However, ever since its market release, the device has been linked to several complications. Many physicians discourage use of the hormonal IUD, despite its popularity. In their opinion, the device will only do more harm than good.
According to court documents, manufacturer Bayer originally marketed the Mirena IUD as a more convenient option for busy women who didn’t want to worry about taking a daily birth control pill. The device is a small, t-shaped, plastic device, which is inserted directly into a woman’s uterus by a healthcare provider. Mirena releases a hormone at a set daily rate for five years. After five years, the device must be removed because it will have expired.
Mirena has been implanted in an estimated 2 million women in the United States and 15 million women worldwide. Since its release, over 47,000 adverse events (any unwanted side effects associated with a prescription drug or medical device) have been reported to the FDA involving Mirena.
Mirena’s label does warn of serious injuries, including pelvic inflammatory disease, ectopic pregnancy (pregnancy outside of the uterus) and perforation upon insertion.




Leave A Comment