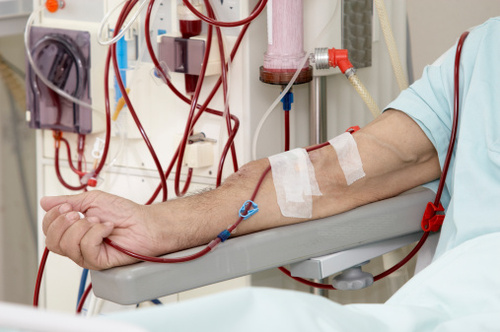
A Florida woman filed a lawsuit against the manufacturers of GranuFlo and NaturaLyte, claiming that the dry acid concentrate administered to her husband at a kidney dialysis center in 2008 led to his death.
The lawsuit was filed in federal court on October 4, 2012. It stated that Fresenius USA Inc. was aware of the serious cardiac risks related to its GranuFlo dialysis treatment when it was first introduced in 2003, but they failed to warn the public of this vital information of GranuFlo’s cardiovascular dangers, heart attacks and death and as a result, they are now responsible for her husband’s death.
GranuFlo and NaturaLyte have become well-known treatment products used in kidney dialysis and hemodialysis. However, these two products have recently been linked to a number of serious side effects. These include heart attacks, irregular heartbeat, death, metabolic alkalosis, low blood pressure, and low blood oxygen.
Fresenius Medical Care is the largest operator of dialysis clinics in the country and provides dialysis treatment to over 400,000 patients each year. According to the FDA, GranuFlo and NaturaLyte have been linked to high levels of bicarbonate in the blood.
By the time Fresenius issued an internal memo to its own physicians and medical directors warning them that use of these two products in the treatment of dialysis increased the risk of cardiovascular events and even death, it was too late for her husband, who stopped breathing after he received a dose of GranuFlo, as part of his three-day-a-week dialysis treatment, the lawsuit says.




Leave A Comment